Overview
Steven W. Levison graduated from the University of Rochester, where he received a B.S. in
Neuroscience in 1983. He completed a Ph.D. in Neurobiology in 1990 at UNC-Chapel Hill, followed by a
postdoctoral fellowship in the Program in Neurobiology and Behavior at Columbia University. Dr. Levison
joined the faculty of New Jersey Medical School in 2004 after 11 years on the faculty of the Penn State
College of Medicine. He presently holds the position of Professor in the Department of Pharmacology,
Physiology and Neuroscience and Directs the Laboratory for Regenerative Neurobiology. Dr. Levison has
authored over 100 peer-reviewed scientific articles and his research has been supported for over 30 years by
the NIH and state and non-profit foundations. He is recognized internationally for his pioneering studies that
have established that there is a regenerative response from the endogenous neural stem cells of the
subventricular zone. Ongoing studies are deciphering how brain development is affected by infections during
pregnancy contributing to neurological and psychiatric disorders. Other studies are manipulating those signals
that regulate neurogenesis, gliogenesis and neuronal survival towards the goal of producing new therapies to
both protect the immature brain from tertiary injuries sustained by hypoxic-ischemic and traumatic brain
injuries and enhance cell replacement and regeneration.
Education
PHD, 1990, University of North Carolina at Chapel Hill, NC
BS, 1983, University of Rochester, Neuroscience, NY
Curriculum Vitae
View CV
Director, Laboratory for Regenerative Neurobiology
Ongoing studies in Dr. Levison's laboratory center focus on the detrimental and beneficial effects of cytokines on
central nervous system development and recovery from injury.
The first major project in the lab is exploring how maternal infections contribute to autism spectrum disorders
(ASD).
Our published data support the hypothesis that elevated levels of IL-6 in the immature brain alter the output of the
secondary neuroepithelial cells of the subventricular zone and the subgranular zone. To test this hypothesis, we
have performed in vitro studies to determine how IL-6 affects the growth of these secondary neuroepithelial cells.
Then, to establish how elevated levels of IL-6 more broadly affect neural development, we have injected postnatal
day 3-7 mouse pups with recombinant murine IL-6. Our studies have demonstrated that this perturbation alters
neurogenesis and gliogenesis. It also produces mice that exhibit multiple deficits in social behaviors, yet they do not
appear to be intellectually impaired. We are presently defining how the transcriptomes of the neural progenitors of
the subventricular zone are altered by IL-6 exposure using bulk and single cell RNAseq. Recently we initiated
collaborative studies with Dr. Peng Jiang at RWJMS to establish how IL-6 alters the development of human neural
progenitors using fused organoids. We are also collaborating with Dr. Ozlem Gunal to understand how perinatal IL-6
treatment affects hippocampal circuitry and hippocampal dependent behaviors. The data obtained from these
studies will lend additional support for the therapeutic neutralization of IL-6 in premature and neonatal infants at risk
for developing ASD.
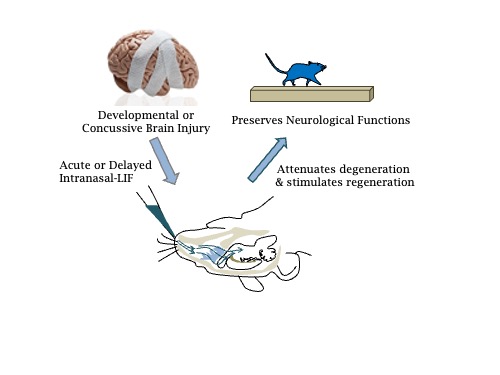
The second major project in the lab is exploring the regenerative and neuroprotective properties of the cytokine
leukemia inhibitory factor (LIF) in recovery from mild head injuries. Our studies are testing the efficacy of intranasally
delivered recombinant LIF to mice during the chronic stage of recovery from a mild TBI and we are performing
experiments to determine the mechanisms through which LIF is reducing delayed neurodegeneration. Completing
these experiments will provide key insights into neurodegenerative processes after brain injury as well as reveal
whether a single cytokine can both protect the brain from injury and amplify the neuroregenerative responses of the
resident neural progenitors to improve functional outcomes.
The final major project in the lab is evaluating how repeated episodes of hypoxia, as experienced by prematurely
born infants, alter the formation of myelin in the central nervous system and whether the LIF can restore normal
myelination. These studies are being performed collaboratively with Dr. Vadim Ten at RWJMS who has developed a
mouse model of repeated hypoxia and in collaboration with Dr. Hiroko Nobuta at RWJMS to test the effects of LIF on
human oligodendrocytes. Our lab also is collaborating with Dr. Terri Wood's lab to establish how repeated episodes
of hypoxia affect mTOR signaling.
